Convert 1 amu to MeV
Atlantic Municipal Utilities (AMU) is located in Atlantic, Iowa and provides the city and surrounding rural area with electric and water service. The Atlantic community has enjoyed the benefits of a not-for-profit, locally owned utility since July 1890. Gram or amu The SI base unit for mass is the kilogram. 1 kilogram is equal to 1000 gram, or 6.949E+26 amu. Note that rounding errors may occur, so always check the results. Neutron = 1 amu. Electron = 1/1837 amu.that is why they generally ignore the mass of electrons in these amu calculations.

One molecule of water (H 2 O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams. The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to.
Solution:
1) Determine mass of 1 amu (in kg):
By definition, the mass of 1 atom of C-12 is 12 amu.Therefore, the mass of one mole of C-12 atoms is, by definition, 12 g
Avogadro's Number is 6.0221409 x 1023 mol¯1
12 g/mol / 6.0221409 x 1023 mol¯1 = 1.9926468 x 10¯23 g (mass of one atom of C-12 in g)
(1.9926468 x 10¯23 g) (1 kg / 1000 g) = 1.9926468 x 10¯26 kg (mass of one atom of C-12 in kg)
1.9926468 x 10¯26 kg/atom / 12 amu/atom = 1.660539 x 10¯27 kg/amu (this is the mass of 1 amu)
2) Use Einstein's mass-energy equation to determine Joules in one amu:
E = mc2E = (1.660539 x 10¯27 kg) (2.99792458 x 108 m/s)2
E = 1.49242 x 10¯10 kg-m2/s2 (this is Joules)
1 Amu Mass
3) Convert J to eV, then MeV
1 eV = 1.60217733 x 10¯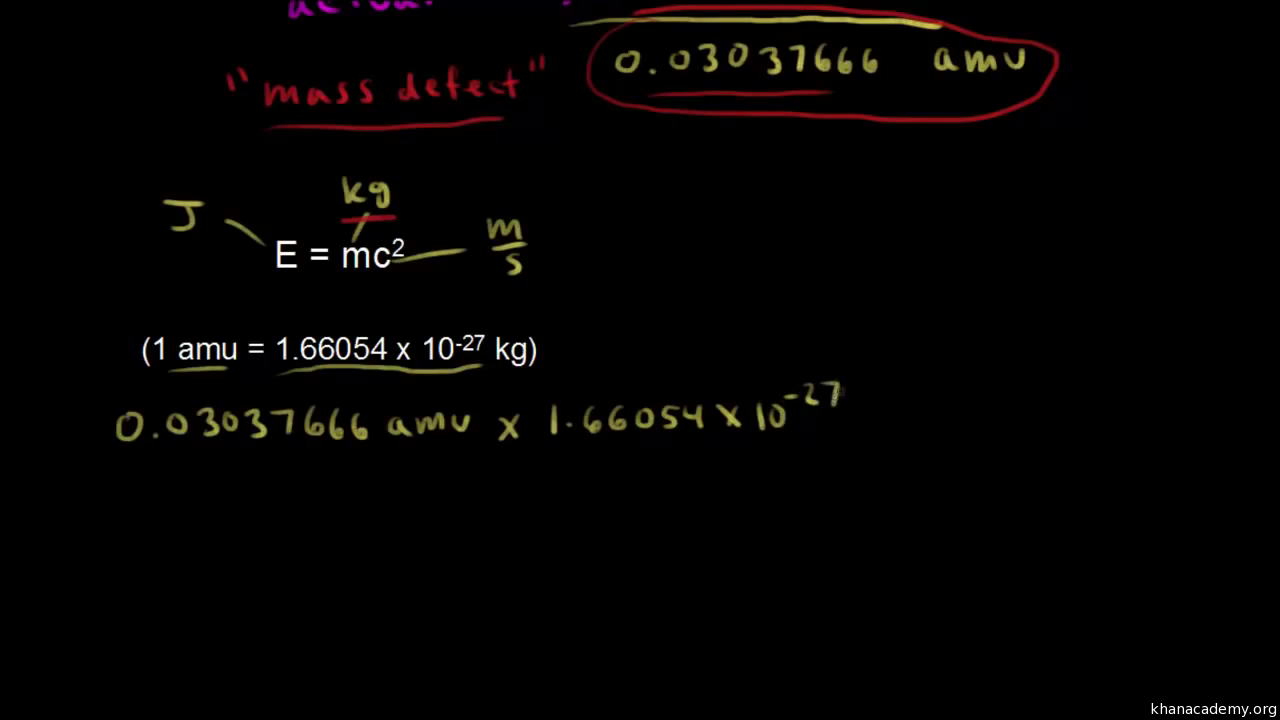 19
19 J
J1.49242 x 10¯10 J / 1.60217733 x 10¯19 J/eV = 931494893 eV
(931494893 eV) (1 MeV / 106 eV) = 931.494893 MeV
Often, 931.5 MeV is the value used in problem solving.
4) 931.5 MeV is the energy in one amu. Is it possible to express the mass of one amu using MeV in some way? I'm glad you asked:
E = mc2931.5 MeV = mc2
m = 931.5 MeV/c2
The use of 931.5 MeV/c2 for the mass of 1 u is wide-spread. So much so that the c2 is often not written and you must infer its presence by context.
5) To sum up:
1 Amu
1 u = 931.5 MeV (expressed as energy)1 u = 931.5 MeV/c2 (expressed as mass)
In solving problems, you may see this unit:
931.5 MeV/u-c2
read it as 931.5 MeV/c2 per 1 u
By the way, if you look around the Internet, you will find values different than the 931.494893 value I calculated. This is because different authors will use values that they have rounded off differently than the ones I used.
For example, I used 1.660539 x 10¯27 kg for the mass of 1 amu. Often, you will see it as 1.66 x 10¯27 kg.
The cumulative effect of these rounding off decisions will be seen in slightly different answers from different authors.
