A mole of water molecules contains 2 moles of hydrogen atoms and 1 mole of oxygen atoms. 2 Sulfuric acid has the chemical formula H 2 SO 4. A certain quantity of sulfuric acid contains 4.89 × 10 25 atoms of oxygen. Conversion Between Moles and Atoms, Molecules and Ions. Using our unit conversion techniques learned in Chapter 1, we can use the mole relationship and the chemical formula to convert back and forth between the moles and the number of chemical entities (atoms, molecules or ions). You are given 850 mg of Ca and asked to find out how many atoms that represents. Each bridge is made as follows: Solution – Ex 1 bridge 1 1000 mg = 1 g bridge 2 40.1 g Ca = 1 mole Ca bridge 3 1 mole = 6.02 x 10 23 atoms. 6.022 x 10²³ Au atoms 1 mole Au atoms. So lets put this value into our formula and see if we can make this work 2.5 mole Au atoms x 6.022 x 10²³ Au atoms 1 1 mole Au atoms. We can cross out the “mole Au atoms” units so the only unit remaining is “Au atoms“.
This online calculator converts moles to liters of a gas at STP (standard temperature and pressure) and liters of gas to moles. The conversion is straightforward and is based on the fact that the ideal gas equation is a good approximation for many common gases at standard temperature and pressure.
Thus, we can rearrange terms in ideal gas equation and write them like this
The relation is the molar volume .
Hence, for a given temperature and pressure, the molar volume is the same for all ideal gases and is known to the same precision as the gas constant: R = 0.082 057 338(47) L atm K−1 mol−1, that is a relative standard uncertainty of 5.7×10−7, according to the 2014 CODATA recommended value1
The molar volume of an ideal gas at standard temperature and pressure (273.15 K, 101.325 kPa) is 22.413 962 x 10-3 m3 mol-1 with standard uncertainty of 0.000013 x 10-3 m3 mol-12
The calculator below uses the formula to convert liters to moles and to convert moles to liters, where is 22.413962
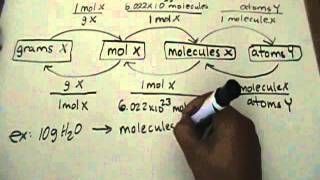
Formula For Atoms To Moles
Molar volume (wikipedia)↩
Molar volume of ideal gas (NIST reference)↩
››Convert mole to atom
Please enable Javascript to usethe unit converter.
Note you can turn off most ads here:
https://www.convertunits.com/contact/remove-some-ads.php
››More information from the unit converter
How many mols in 1 atoms?The answer is 1.660538863127E-24.
We assume you are converting between mole and atom.
You can view more details on each measurement unit:
mols oratoms
The SI base unit for amount of substance is the mole.
1 mole is equal to 6.0221415E+23 atoms.
Note that rounding errors may occur, so always check the results.
Use this page to learn how to convert between moles and atoms.
Type in your own numbers in the form to convert the units!
››Quick conversion chart of mols to atoms
1 mols to atoms = 6.0221415E+23 atoms
2 mols to atoms = 1.2044283E+24 atoms
3 mols to atoms = 1.80664245E+24 atoms
4 mols to atoms = 2.4088566E+24 atoms
5 mols to atoms = 3.01107075E+24 atoms
6 mols to atoms = 3.6132849E+24 atoms
7 mols to atoms = 4.21549905E+24 atoms
8 mols to atoms = 4.8177132E+24 atoms
9 mols to atoms = 5.41992735E+24 atoms
Moles To Atoms Ag
10 mols to atoms = 6.0221415E+24 atoms
››Want other units?
You can do the reverse unit conversion fromatoms to mols, or enter any two units below:
Moles To Atoms Chart
››Common amount of substance conversions
mols to millimol
mols to centimol
mols to picomol
mols to molecule
mols to micromol
mols to nanomol
mols to decimol
mols to kilomol
››Definition: Mole
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is 'mol.'
››Definition: Atom
Moles To Atoms Practice
This site uses an exact value of 6.0221415 x 1023 for Avogadro's number. This is the number of atoms in 1 mole of a chemical element.

››Metric conversions and more
ConvertUnits.com provides an onlineconversion calculator for all types of measurement units.You can find metric conversion tables for SI units, as wellas English units, currency, and other data. Type in unitsymbols, abbreviations, or full names for units of length,area, mass, pressure, and other types. Examples include mm,inch, 100 kg, US fluid ounce, 6'3', 10 stone 4, cubic cm,metres squared, grams, moles, feet per second, and many more!
